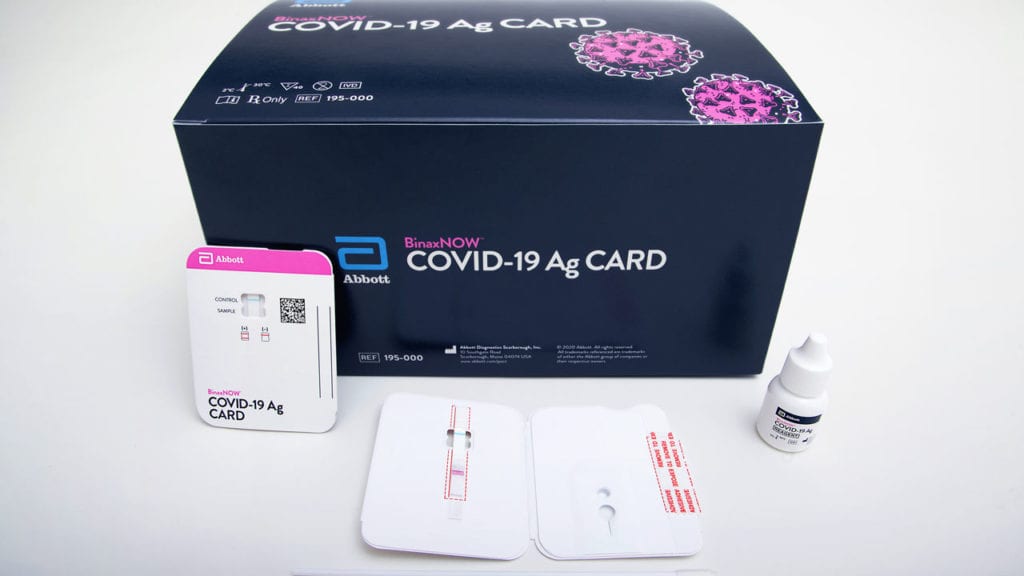
The FDA issued Emergency Use Authorization on August 26, 2020 for Abbott’s BinaxNOW COVID-19 Ag Card rapid test for detection of the COVID-19 infection. This simple, inexpensive, accurate test will help to eliminate testing delays. Unlike other COVID-19 diagnostics, it requires no special equipment. Abbott says it will sell for $5 and provide results in 15 minutes.
Charles Chiu, M.D., Ph.D., professor of Laboratory Medicine at the University of California, San Francisco said, “Our nation’s frontline healthcare workers and clinical laboratory personnel have been under siege since the onset of this pandemic. The availability of rapid testing for COVID-19 will help support overburdened laboratories, accelerate turnaround times, and greatly expand access to people who need it.”
Using methods utilized for detecting HIV and Influenza, the new test detects antigens, which are viral proteins that elicit an immune response and are unique to SARS-CoV-2, the virus that causes COVID-19. Abbott’s diagnostic isn’t the first antigen test for SARS-CoV-2 on the market. However, other antigen tests require specialized equipment to obtain the results, thus making them less suitable for widespread use.
Michael Mina, an epidemiologist at Harvard University’s T.H. Chan School of Public Health, who has long been an advocate for antigen testing, said “This is wonderful news. It will be administered first by physicians as a diagnostic, but this is a big step towards the development and approvals of similar assays that can eventually be used over the counter as public health tools. I am very excited about this milestone.”
BinaxNOW uses a credit card–size device to detect SARS-CoV-2 antigens. A health care professional performs a nasal swab of the patient, then inserts the swab into the device along with a few drops of solution, allowing the sample to flow across a strip containing antibodies that will bind to the viral material thus causing the strip to change color, which indicates the presence of SARS-CoV-2 proteins.
Antigen tests, though much quicker than PCR tests, have been proven to be inaccurate, most notably producing a high percentage of false-negative results. However, in data submitted to the FDA from a clinical study conducted by Abbott with several leading U.S. research universities, the BinaxNOW COVID-19 Ag Card demonstrated 97.1 percent accuracy for positive results and 98.5 percent accuracy for negative results in patients suspected of COVID-19 by their healthcare provider within the first seven days of symptom onset.
Abbott will ship tens of millions of tests in September, ramping up to 50 million tests a month at the beginning of October. The company has invested hundreds of millions of dollars since April in two new U.S. facilities to manufacture BinaxNOW at a massive scale.
Abbott is also offering a free mobile app that will allow people to display their results obtained through a healthcare provider when entering facilities requiring proof of testing. If test results are negative, the app will display a digital health pass via a QR code, similar to an airline boarding pass. If test results are positive, people will receive a message telling them to quarantine and to talk to their doctor. As they’re required to do for all COVID-19 tests, healthcare providers in all settings will be required to report positive results to the CDC and other public health authorities, regardless of whether or not they use the app. The digital health pass is stored in the app temporarily and expires after the time period specified by organizations that accept the app.
The NAVICA app is optional and an easy-to-use tool that allows people to store, access, and display their results with organizations that accept the results so people can move about with greater confidence. The app is supported by Apple and Android digital wallets and will be available from public app stores in the U.S.
Robert B. Ford, president and chief executive officer of Abbot, said, “BinaxNOW and the NAVICA app give us an affordable, easy-to-use, scalable test, and a complimentary digital health tool to help us have a bit more normalcy in our daily lives.”